The primary difference between the designations of cosmetics and OTC (over-the-counter drugs) is the intended use of the product, based on the brand’s claims, consumer perception and ingredients.
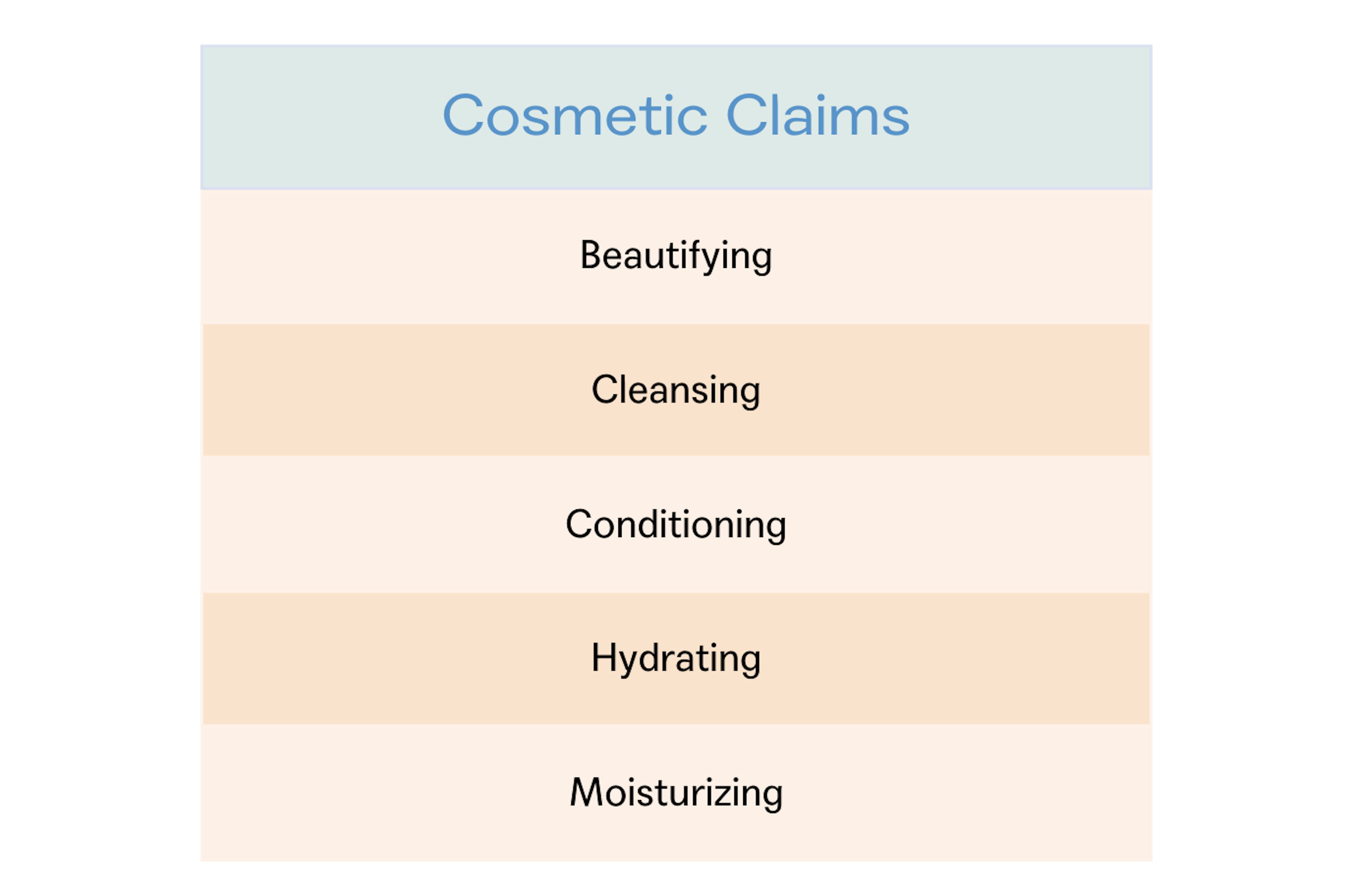
The FDA defines a cosmetic as a product, except soap, “intended to be applied to the human body for cleansing, beautifying, promoting attractiveness, or altering the appearance.” Among the products included in this definition are skin moisturizers, perfumes, lipsticks, fingernail polishes, eye and facial makeup, cleansing shampoos, permanent waves, hair colors, and deodorants. The law does not require FDA approval of cosmetics before they go on the market unless they have color additives.
In the US, a product qualifies as a drug when the product is intended for a therapeutic use, such as diagnosing, mitigating, treating, curing or preventing disease, or to affect the structure or function of the body. Drugs can be marketed as OTC if they comply with established OTC monographs established by the FDA, or through the submission of an NDA (New Drug Application) to the FDA (Learn more: How OTC Drug Products are Approved by the FDA). Examples of OTC drug products include, sunscreen, antidandruff shampoo, acne care, and hand sanitizer.
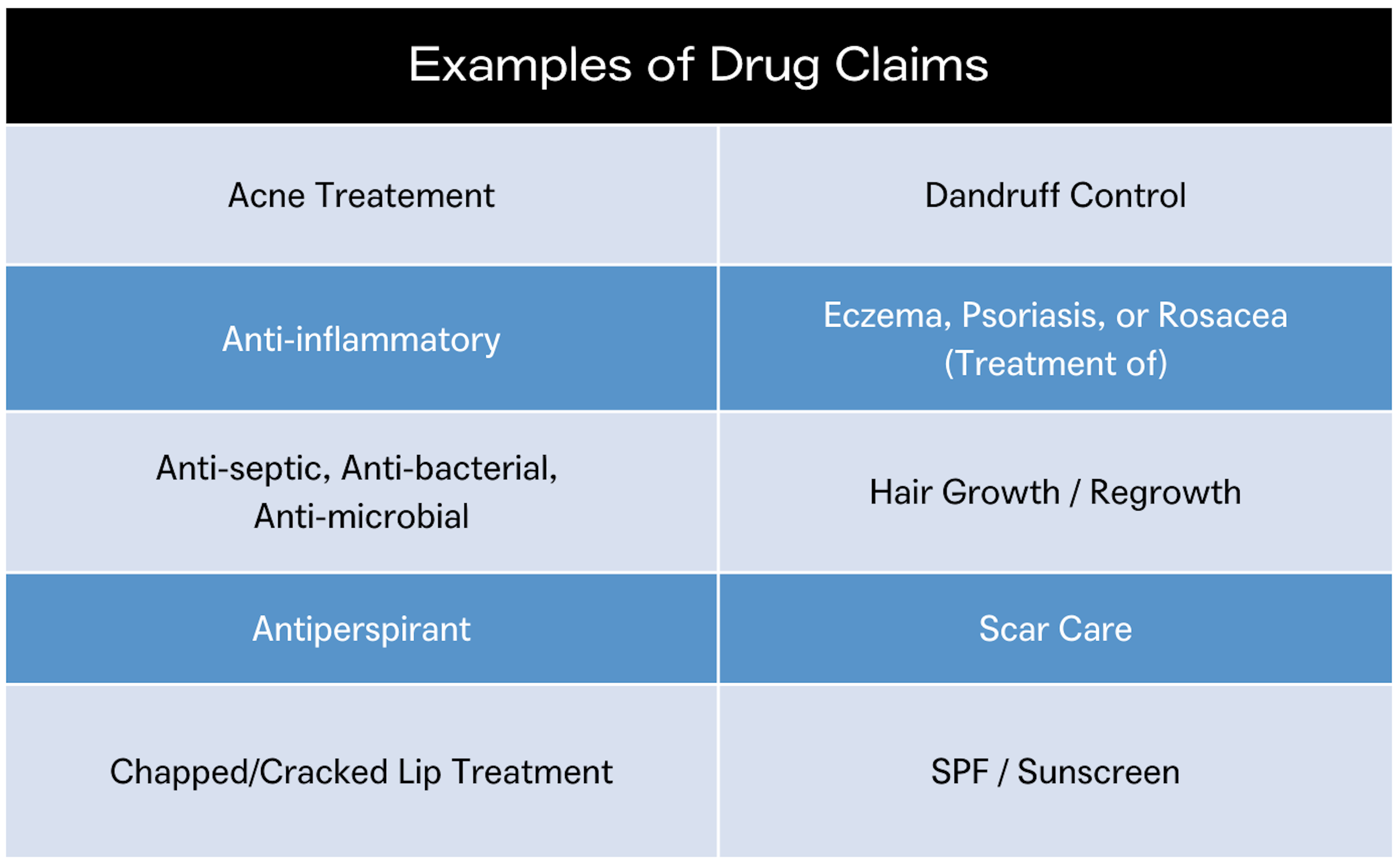
These designations are not mutually exclusive as a product can be both cosmetic and OTC when it has two or more intended uses. For example, an anti-dandruff shampoo will claim to both cleanse hair and treat dandruff. Additional examples include makeup with sun protection claims and deodorants that are also antiperspirants. These products must comply with the requirements for both cosmetics and drugs.
Regardless of whether a product is OTC or cosmetic, it is up to companies to ensure that their products claims are truthful. Once a product hits the market, government agencies can still review product claims and take action against companies suspected of making claims that are untruthful or misleading.
Establishing Intended Use
Intended use is established based on the product’s claims, consumer perception and ingredients. A product’s claims are assertions made by the brand in promotion of the product’s benefits. They are found on the product labeling, in advertising, on social media, or in other promotional materials. Consumer perception, or the product’s reputation, involves asking why the consumer is buying a product and what the consumer expects it to do. Product claims can be a primary influence in shaping consumer perception which is why they are closely scrutinized when establishing intended use.
Ingredients can also cause a product to be considered a drug because they have a well-known therapeutic use like fluoride (prevents tooth decay). If levels of the ingredient are lower than the defined amount in a relevant monograph, then the product cannot be marketed as an OTC drug. For example, for a product to claim “acne treatment” through salicylic acid as an active ingredient, it must have levels between 0.5% to 2% of salicylic acid. If it is less than .5%, then the product may only make cosmetic claims that speak to cosmetic benefits like exfoliation or smoothing.
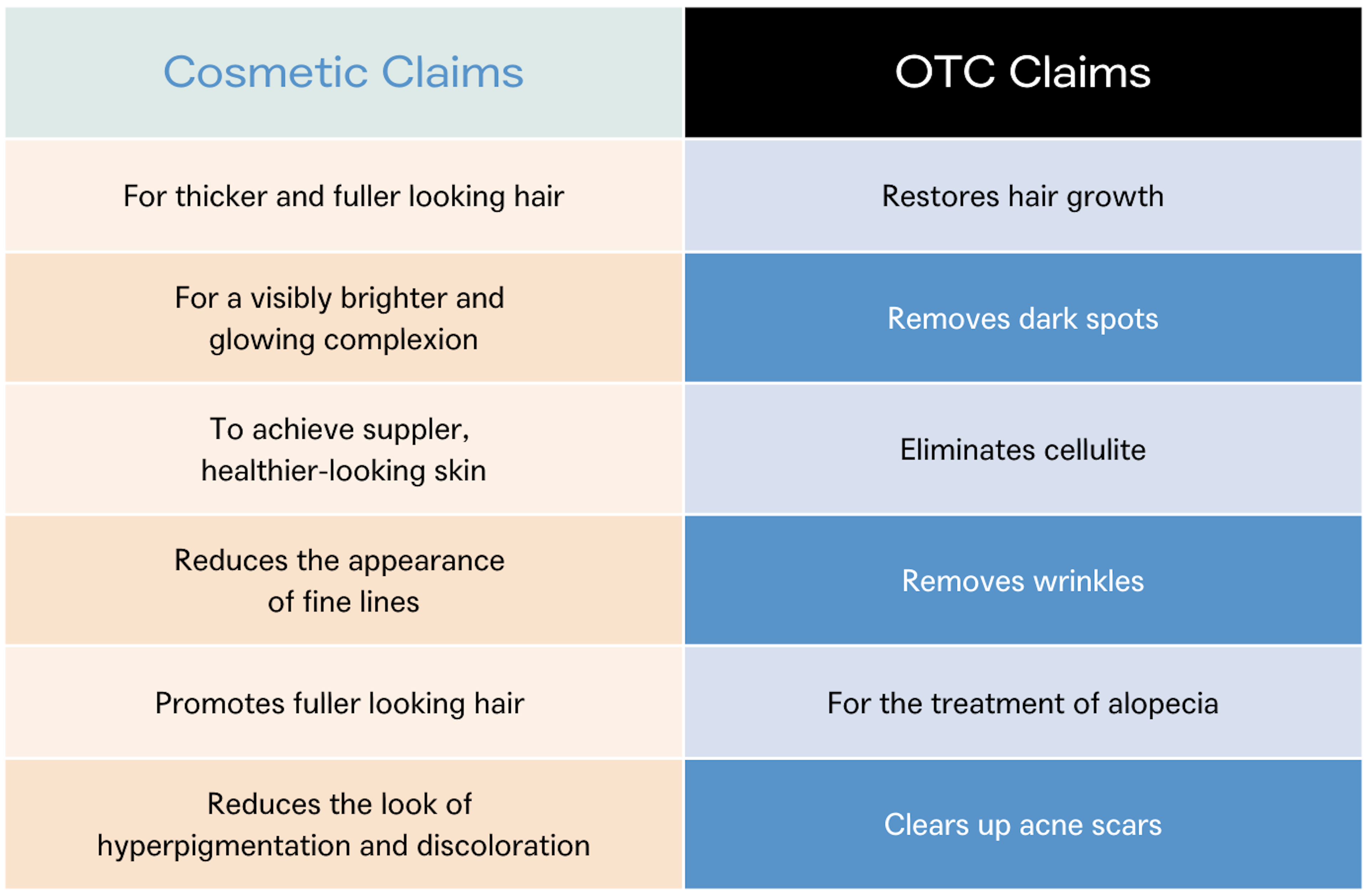
The differentiation can be difficult to decipher, and brands need to be mindful of the language used in the marketing of the product. If a product is intended to make lines and wrinkles less noticeable by moisturizing the skin (lotion) or hiding them (makeup), then it qualifies as a cosmetic claim. But if a product claims to remove wrinkles or increase the skin’s production of collagen, then it would be considered a drug by the FDA.
Learn more about OTC drug products:How OTC Drug Products are Approved by the FDA
Prime Matter Labs offers tailored product development and production, adapting and innovating along with your business. Work with our team to capture exactly what it's going to take to meet your consumers’ unique needs. Contact your Prime Matter Labs Project Manager or start your project here.