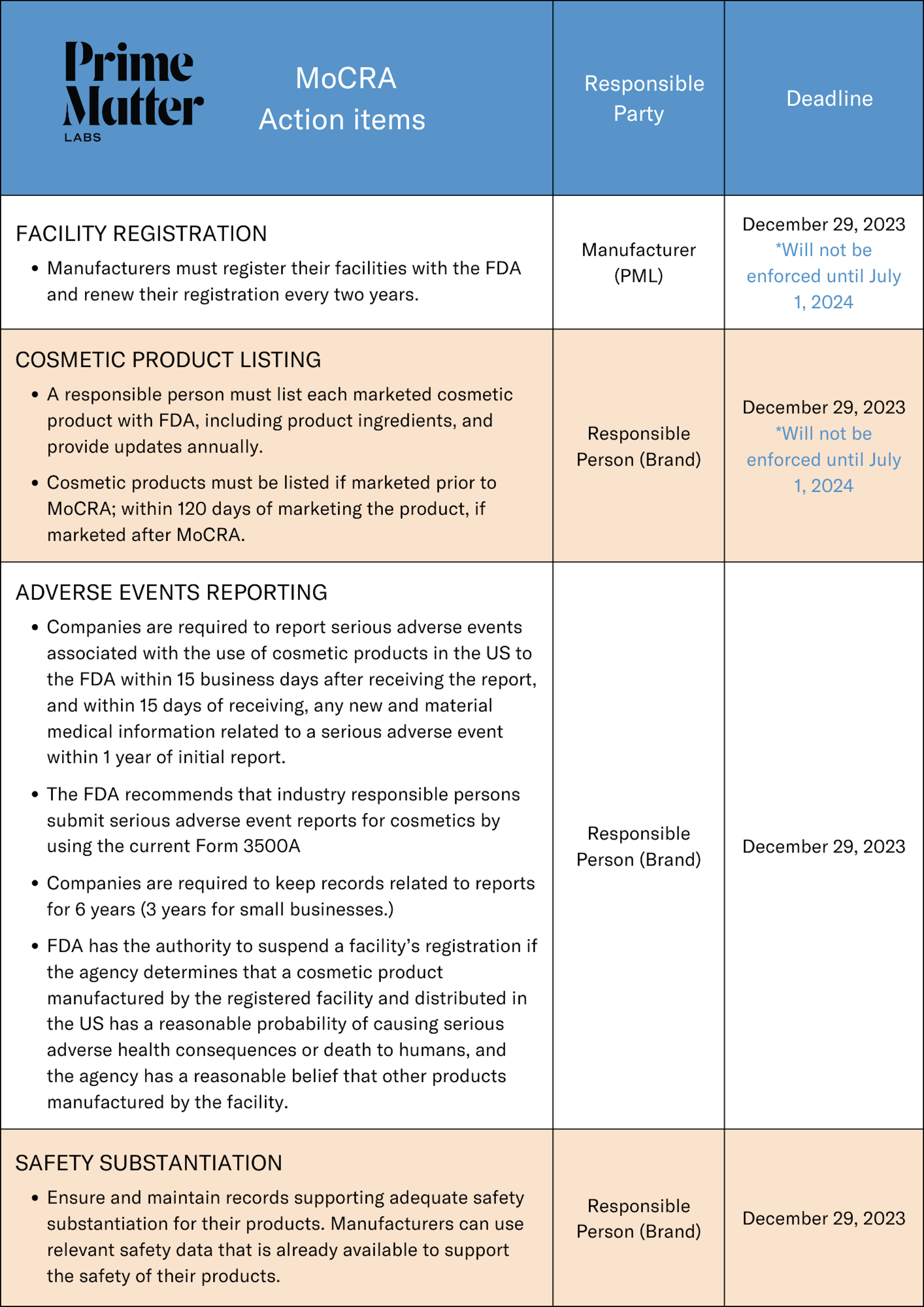
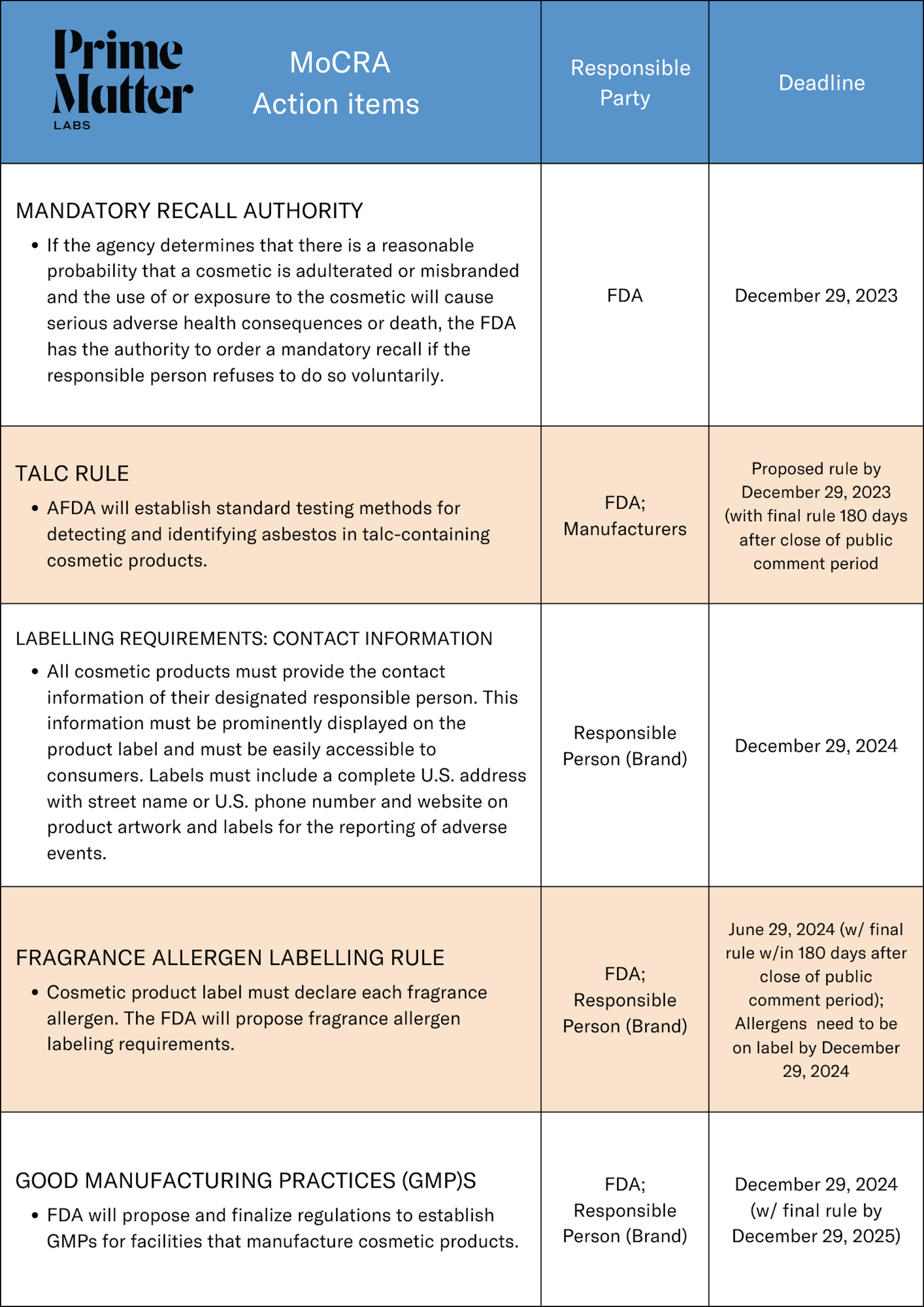
Beginning on December 29, 2023, the Modernization of Cosmetic Regulation Act of 2022 (MoCRA) will begin to take effect, helping to ensure the safety of cosmetic products for all consumers. The legislation makes some important changes to the current regulatory framework for cosmetics, including mandating the registration of facilities, cosmetic product listing, serious adverse event reporting and recordkeeping, and safety substantiation. Additionally, it gives the U.S. Food & Drug Administration (FDA) the authority to order a mandatory recall of a cosmetic product and to suspend a facility registration if FDA determines there are serious adverse health concerns.
Signed by President Biden in December 2022 as part of the 2023 Omnibus Bill, the act gave manufacturers a one-year window to comply with the mandates. The legislation also gives an extended deadline for compliance with new fragrance allergens and contact information labeling requirements, and standard testing methods for detecting asbestos in talc-containing cosmetic products.
On November 8th, 2023, the FDA announced it will delay the enforcement of cosmetic product facility registration and cosmetic product listings, extending the timeline by additional six months to July 1, 2024 to ensure the industry has sufficient time to submit information. The FDA has advised that it will be ready to accept registration and listing information by the original deadline of December 29, 2023, and encourages companies to meet that deadline if they are able to do so.
Additionally, the FDA will enact new regulations establishing required good manufacturing practices (GMPs). GMPs are a set of guidelines covering all aspects of the manufacturing process to ensure the safety and quality of products. The FDA has conducted a virtual listening session and taken comments from cosmetic manufacturers, consumer organizations, and other experts to inform the FDA’s development of the regulations. They will deliver the proposed rule by December 29, 2024, with a final rule no later than end of December 29, 2025. Once the final rule is established, all facilities where cosmetics are manufactured will need to comply or be modified to comply by the deadline. This could be a challenge for some manufacturers to complete within the time frame as they may need to upgrade facilities, equipment, processes, and personnel.
Prime Matter Labs complies with Current Good Manufacturing Practice (CGMP) per 21 CFR 210 and 211, which is the main regulatory standard for ensuring pharmaceutical quality products in the United States. This includes establishing strong quality management systems, obtaining quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories. This means we already have the equipment, processes, and personnel in place for following the FDA's GMPs for drugs, which cover all aspects of the manufacturing process, from the procurement of raw materials to the packaging and distribution of finished products. Additionally, our facilities are equipped for the manufacturing of over-the-counter (OTC) drug products like sunscreens, acne treatments, and anti-perspirant. (View all of our Certifications here.)
Exemptions
Small businesses, defined as businesses with average gross annual sales of cosmetics in the US for the previous three-year period of less than $1,000,000, are exempt from the GMPs, facility registration, and product listing requirements. However, they are still required to maintain adverse event reports for three years.
Exemptions do not apply to manufacturers or facilities that manufacture or process the following cosmetic products:
- Products that regularly come into contact with mucus membrane of the eye.
- Products that are injected.
- Products that are intended for internal use.
- Products that are intended to alter appearance for more than 24 hours under customary or usual conditions of use and removal by the consumer is not part of such conditions of use.
MoCRA 2022 Preparation for Brands
Brands should start planning and implementing their compliance strategies to ensure that they meet MoCRA requirements before they take effect on December 29, 2023:
Cosmetic Product Listing: Brand must list each marketed cosmetic product with FDA and provide any updates annually. While brands will be able to begin complying on December 29, 2023, the FDA will not enforce until July 1, 2024.
Brands will need to provide product name, brand name, responsible person, product registration number, ingredient list, date of first marketing and any other info required by the FDA. Brands should start preparing now by collecting the required information and developing a process for updating their product listings annually.
UPDATE: On December 18, 2023, the FDA launched its Cosmetics Direct electronic submission portal for the registration and listing of cosmetic product facilities and products. While the FDA is also developing paper forms as an alternative submission tool (available soon), the agency strongly encourages electronic submissions to facilitate efficiency and timeliness of data submission and management for the agency.
Labelling: Brands will need to review current labeling practices and prepare to submit detailed product listings, including ingredients, for all cosmetic products marketed prior to and post MoCRA enactment, as well as establishing a process for regular updates.
Safety Substantiation: Brands should begin to gather materials that provide evidence to support the safety of a cosmetic product. This evidence can include data from clinical studies, toxicology tests, and other scientific research that is already available.
Adverse Events: Adverse events are defined by the FDA as “any health-related event associated with the use of a cosmetic product that is adverse” and may result in death, inpatient hospitalization, or significant disability or disfigurement. Brands will need to identify the designated “responsible person,” familiarize themselves with the process for reporting adverse events and establish a plan to report within 15 business days of notification by the designated responsible person. The responsible person, defined as the person “whose name appears on the label of such cosmetic product,” must include a copy of the label on or within the retail packaging of such cosmetic product.
If the responsible person receives medical or other information about the adverse event within 1 year of the initial report to FDA, they must submit this new information to FDA within 15 business days. FDA will also have access to adverse event reports during an inspection. FDA recommends that industry responsible persons submit serious adverse event reports for cosmetics by using the current Form 3500A
Talc Rule: By December 29, 2023, the FDA is to issue a proposed rule establishing standardized talc testing methods and requiring testing of talc-containing products for the presence of asbestos. Once the rules are established, brands should work with their contract manufacturers to ensure that ingredients and products are properly inspected and tested and adhere to the established rules.
By December 29, 2024, brands will need to update their labels to disclose any known fragrance allergens in their products and provide the contact information of their designated responsible person.
Labelling: Brands will now be required to clearly identify each fragrance allergen on the product label by the December 29, 2024 deadline. The FDA has not yet finalized the list of fragrance allergens but the proposed rulemaking for allergen identification is to be issued by FDA no later than June 29, 2024. The fragrance allergens identified by other countries’ regulatory bodies can help guide brands on the ingredients likely to be identified by the FDA as they prepare for the deadline.
Additionally, all cosmetic products must provide the contact information of their designated responsible person. The information must be prominently displayed on the product label and easily accessible to consumers. Labels must include a complete U.S. address with street name or U.S. phone number and website on product artwork and labels for the reporting of adverse events.
By December 29, 2025, brands will need to ensure that their products are being manufactured in a facility that adheres to the new Cosmetic GMPs as established by the FDA.
GMPs: The FDA will propose regulations by December 29, 2024, with final rule by December 29, 2025, to establish GMPs for facilities that manufacture cosmetic products. Brands can start getting ahead of this now by checking with their current manufacturers to make sure that they are prepared for the regulations or working to identify manufacturers that are already following GMPs standards like Prime Matter Labs.
The Prime Matter Labs Quality and Regulatory team has the experience, knowledge, and equipment to help our brand partners navigate required testing and procedures and ensure that delivered products comply with federal regulations. With one of the broadest sets of certifications and full in-house testing capabilities, our facility is one of the few in the country that can truly deliver your entire personal care line end-to-end.
To learn more about how Prime Matter Labs can help your brand comply with MoCRA 2022, reach out to your current project manager, email help@primematterlabs.com or start your project here.
Download the full guide in pdf form HERE.