Acne is the most common skin condition in the US, according to the American Academy of Dermatology Association. It’s estimated that about 85% of people will get acne at some point in their lives. And while you may assume that acne is primarily the scourge of hormonal teens, acne can occur at any stage of life and may continue into a person’s 30s and 40s, especially for women. According to The Journal of Clinical and Aesthetic Dermatology, 22% of adult women are affected by acne, compared to less than 5% of adult men, which may be due to hormones fluctuating throughout their cycles and lives.
ACNE TREATMENT
Acne treatment products are considered drugs by the FDA. Certain acne treatments qualify as over-the-counter (OTC) drugs according to the FDA, and these must comply with the OTC monograph for Topical Acne Drug Products. OTC monographs are essentially rules or guidelines for acceptable ingredients, dosage strength, dosage form, formulation, claims guidance, and labeling necessary and appropriate for the safe and effective use of that drug. If the product complies with the conditions of the monograph, premarket approval is not necessary.
Acne is defined by the FDA as a “disease involving the oil glands and hair follicles of the skin which is manifested by blackheads, whiteheads, acne pimples, and acne blemishes.” It is often caused by the overproduction of sebum which can clog pores. As the pores become clogged with excess oil and dead skin cells, they can become inflamed and infected and may lead to the formation of pimples. Hormones can also contribute to the development of acne by increasing oil production, promoting inflammation, and altering the balance of bacteria on the skin. In some cases, bacteria called Cutibacterium acnes or C. acnes (previously Propionibacterium acnes or P. acnes) can also contribute to the development of acne. While C. acnes is the primary bacteria associated with acne, other types of bacteria can also contribute to the development of the condition.
Learn more about How OTC Drug Products are Approved by the FDA here.
ACTIVE INGREDIENTS
There are currently five active ingredients that qualify for OTC topical acne treatment according to the monograph: benzoyl peroxide, resorcinol, resorcinol monoacetate, salicylic acid, and sulfur. Benzoyl peroxide, salicylic acid and sulfur can be used on their own as anti-acne actives, but resorcinol and resorcinol monoacetate can only be used in combination with sulfur.
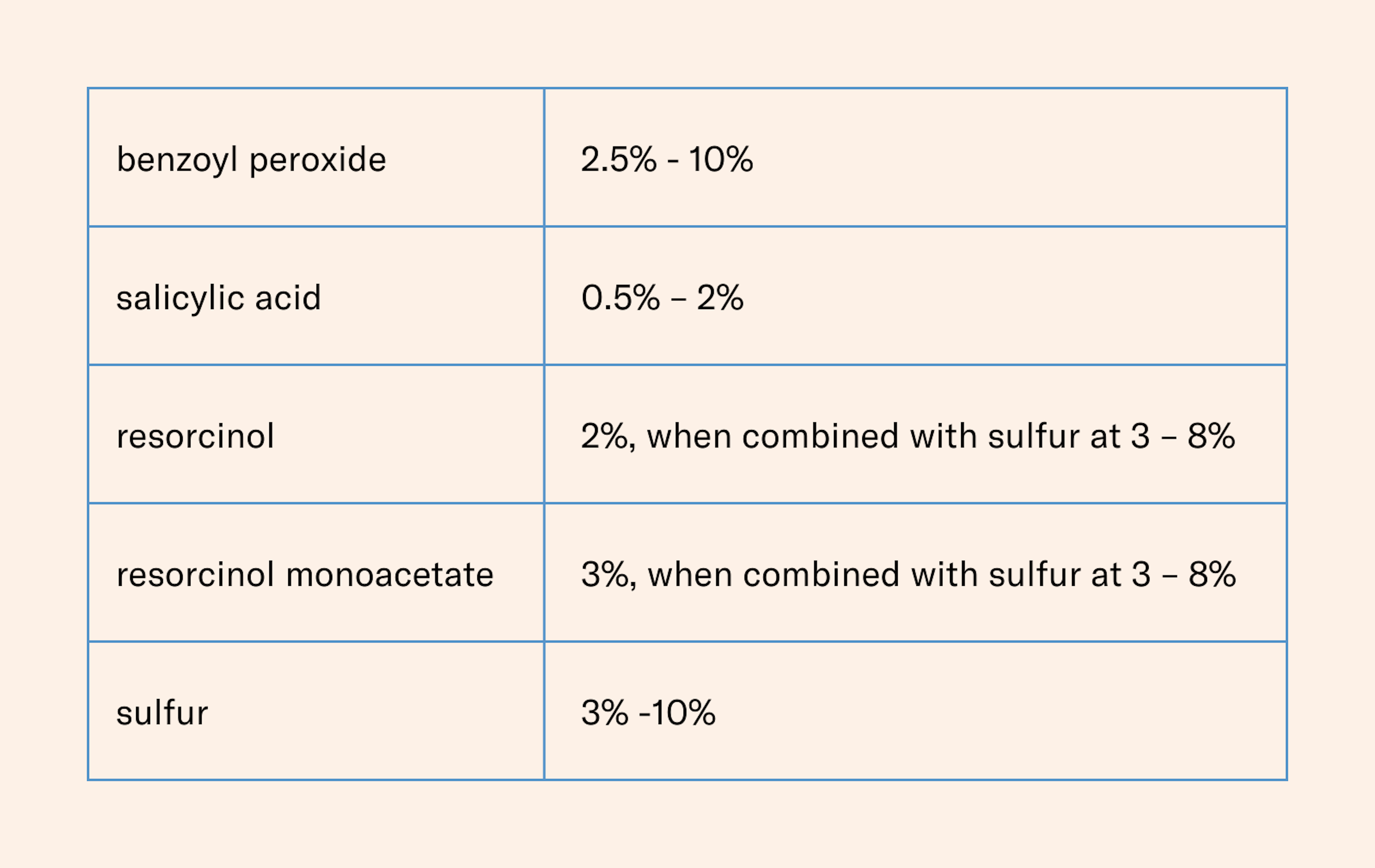
Benzoyl peroxide and salicylic acid are the two most common and well-known active ingredients for acne treatments, but all the ingredients are keratolytic agents with varying degrees of antimicrobial properties.
Keratolytic is a term used to describe a substance that can break down or dissolve the protein keratin. By breaking down the keratin protein, these ingredients can help to loosen and remove the dead skin cells and other debris that can clog pores and lead to the development acne. The antimicrobial properties of the ingredients work to kill or inhibit the growth of C. acnes bacteria to help to reduce the number of bacteria on the skin and prevent the development of new acne lesions.
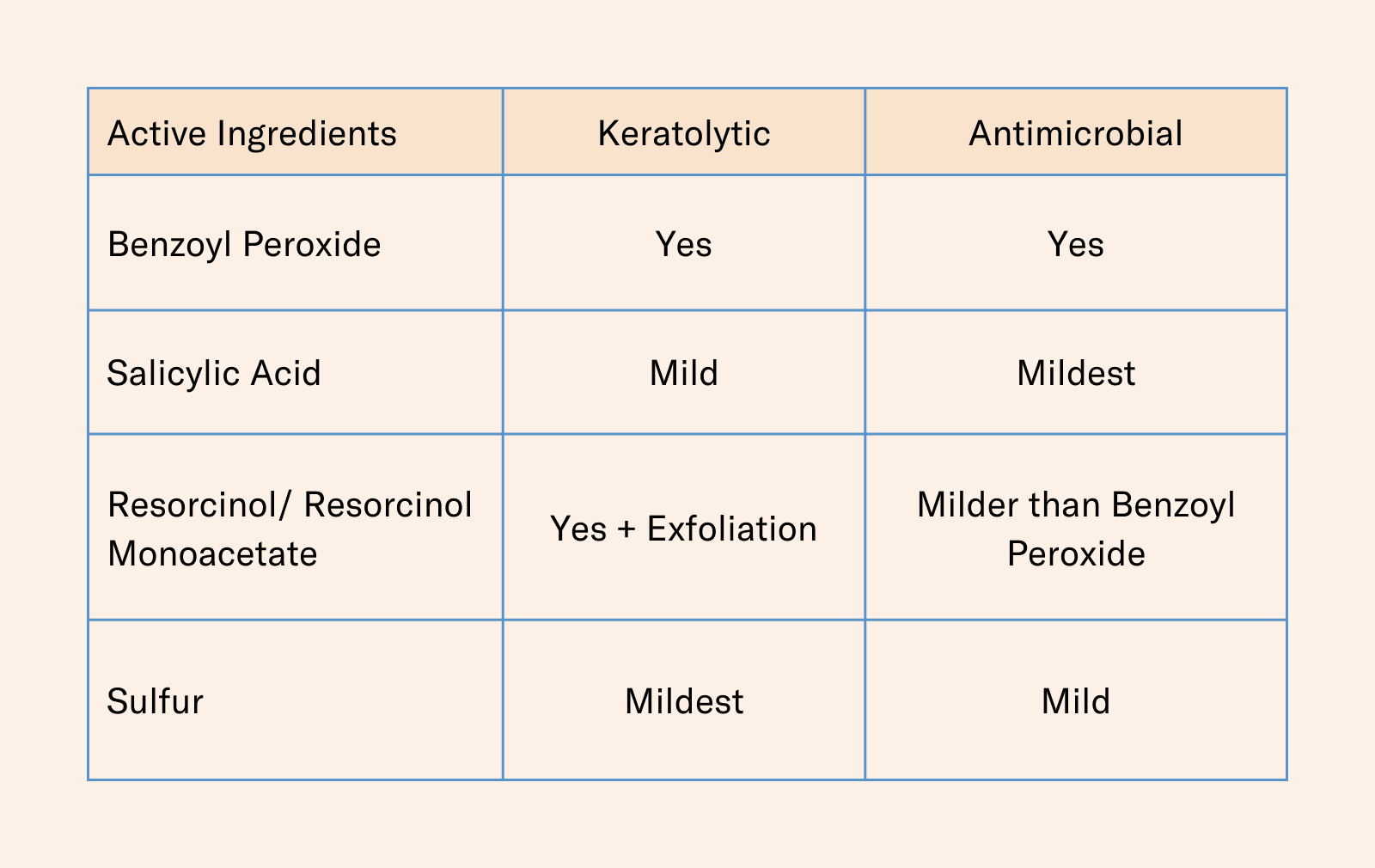
Benzoyl peroxide is the most keratolytic of these ingredients. Salicylic acid also has some keratolytic properties, but it is milder than benzoyl peroxide. In terms of C. acnes, benzoyl peroxide is the most effective while salicylic acid is considered to have milder antimicrobial effects.
Benzoyl Peroxide: Benzoyl peroxide is considered the most effective in combating acne of all the ingredients listed in the monograph, but it is also known to be the harshest on the skin. It is effective against the bacteria that contributes to the development of acne (C. acnes) and is an effective keratolytic agent. The ingredient can present some challenge to formulators as it decomposes upon exposure to heat, light, and air. This means that formulations containing benzoyl peroxide must be carefully stabilized to prevent decomposition and maintain efficacy.
Salicylic Acid: A beta-hydroxy acid that works by exfoliating the skin and helping to unclog pores. Known for its exfoliating abilities, salicylic acid is milder than benzoyl peroxide and helps to prevent the buildup of dead skin cells that can contribute to the development of acne. While salicylic acid is not primarily used for its antimicrobial properties, it can help to reduce the number of bacteria on the skin by removing the debris that can provide a food source for the bacteria.
Resorcinol: It is not as widely used as other acne treatments and may be more likely to cause skin irritation in some people. As a keratolytic agent, it helps to break down the keratin that can block pores and cause pimples. It can only be used in combination with sulfur, according to the monograph.
Resorcinol Monoacetate: Like resorcinol, resorcinol monoacetate works by breaking down keratin and reducing the formation of new acne lesions. It is a derivative of resorcinol that is more stable, although it can still be sensitive to light, air, and heat. It is less irritating to the skin than the unmodified form of resorcinol and has some antioxidant properties, which can help to protect the skin from free radical damage.
Sulfur: Known to be the mildest, yet also the most pungent in scent, of all the ingredients, sulfur is often combined with other active ingredients, such as salicylic acid or benzoyl peroxide, to enhance its effectiveness.
LABELING
The “statement of identity” is the name that appears on the OTC product. This includes the drug's established name and a statement of its general pharmacological category or its intended principal action. For acne, the products must be identified as an “acne medication” or “acne treatment.” The phrases “acne medication” or “acne treatment” should be combined with the dosage form e.g., cream, gel, lotion or ointment.
In addition to the statement of identity, the monograph dictates the language that can be featured on the product label for uses, warnings and directions.
While the monograph outlines how formulators should approach specific anti-acne actives and labeling, it still leaves room for chemists to innovate with formulas that can also provide cosmetic benefits. The Prime Matter Labs R&D team is experienced in collaborating with brand partners to develop acne treatment products that provide both impactful results as well as benefits and features that reflect the trends in the category. Reach out to your Prime Matter Labs project manager for more info on our innovation products or start your project here.
Learn more about The Difference Between Cosmetic and OTC Designations here.